干扰素(Interferon,IFN)受体蛋白是宿主细胞分泌的一类细胞因子,可调节免疫应答。作为被发现的第一类细胞因子,它被命名为“干扰素”,因为这种蛋白质能够干扰病毒的复制。当存在病原体时,通常由宿主细胞释放干扰素,周围未感染的细胞感知后激活适当的细胞防御机制,以便消除病原体。IFN细胞因子根据其结合的不同干扰素受体分为三种类型(I型、II型和III型);每种IFN细胞因子诱导一种特定的免疫反应。此外,IFN细胞因子介导信号会促进主要组织相容性I类和II类分子(MHC I,MHC II)的上调,并活化许多下游信号级联,从而生成抗病毒防御机制。
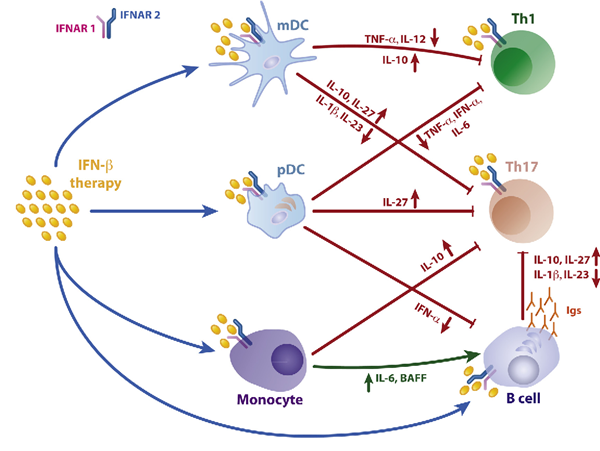
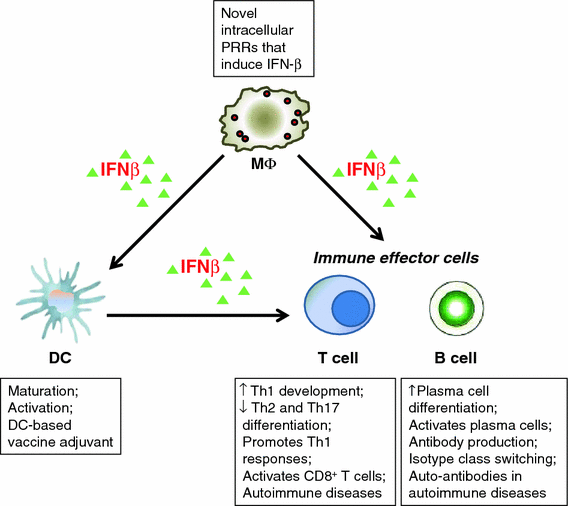
干扰素β(Interferon beta,IFN-β,IFNb)是细胞中第一波细胞因子反应的一部分。病原体感染可导致干扰素调节因子3(IRF3)的激活,该因子以反式激活IFN-β基因转录。与其他干扰素相比,IFN-β在生物学上是独特的,研究表明,与IFN-α相比,IFN-β具有重叠和不同的基因表达模式。似乎IFN-β以比其他I型IFN更高的亲和力与I型IFN-受体结合,并且它还可以以不同的方式调节受体内化。此外,IFN-β长期以来一直被认为可以抑制病毒复制,作为身体先天抗病毒反应的一部分,并被用作治疗多发性硬化症(MS)和一些肿瘤的药物。
我们推荐PBL Assay Science的高灵敏度的人IFN-β ELISA试剂盒,测量人血清、血浆和组织培养基中的IFN-β水平。作为PBL Assay Science在中国区域的代理商,艾美捷科技将为中国客户提供全面的PBL Assay Science产品以及客户订制化服务。欢迎大家随时联系我们。

产品货号 | 产品名称 | 样本类型 |
41415 | VeriKine-HS Human Interferon Beta Serum ELISA Kit | 血清、血浆、组织培养基(TCM) |
VeriKine HS人IFN-β ELISA试剂盒设计用于测量自身免疫性疾病血清、健康血清/血浆或组织培养基样品中低水平或基础水平的人IFN-β,LLOQ灵敏度为1.2 pg/ml。
该测定法适用于测量人类血清样品中的商标治疗分子。研究人员和临床研究人员检查a)IFN-β分子的药代动力学,b)作为生物标志物的IFN-β,或c)作为TLR制剂或其他免疫反应调节剂活性的药效学标志物的IFN-β,将发现这种免疫测定是一种重要的实验室工具。
人IFN-β ELISA试剂盒(货号:41415) | |
样品类型 | 血清、血浆、组织培养基 |
特异性 | 人IFN-β |
检测范围 | Protocol A: 1.2 - 150 pg/ml(提高血清性能) Protocol B: 2.3 - 150 pg/ml |
灵敏度(LLOQ) | 1.2 pg/ml |
实验时间 | Protocol A: 3小时30分 Protocol B: 3小时 |
变异系数 | Inter-Assay < 8% Intra-Assay < 10% Spike Recovery > 90% in Serum |
标准曲线 |
|
引用文献(Citations):
1. Lei, X. et al., (2024), "CD4+T cells produce IFN-I to license cDC1s for induction of cytotoxic T-cell activity in human tumors", Cell Mol Immunol., PMID: 38383773, DOI: 10.1038/s41423-024-01133-1 (link)
2. Morihiro, K. et al., (2024), "RNA Oncological Therapeutics: Intracellular Hairpin RNA Assembly Enables MicroRNA-Triggered Anticancer Functionality", J Am Chem Soc. PMID: 38170469, DOI: 10.1021/jacs.3c09524 (link)
3. Rohilla, A. et al., (2023), "Structure-based virtual screening and in vitro validation of inhibitors of cyclic dinucleotide phosphodiesterases ENPP1 and CdnP", Micro Spectr., e0201223, PMID: 38095464, DOI: 10.1128/spectrum.02012-23 (link)
4. Smith, KER., et al., (2023), "A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanomia: safety, efficacy, and T cell responses", Front Immunol., 14:1279387, DOI: 10.3389/fimmun.2023.1279387 (link)
5. Grunhagel, B., et al., (2023), "Reduction of IFN-I Responses by Plasmacytoid Dendritic Cells in a Longitudinal Trans Men Cohort, iScience, DOI: 10.1016/j.isci.2023.108209 (link)
6. Nilsen, K.E., et al., (2023), "Peptide derived from SLAMF1 prevents TLR4-mediated inflammation in vitro and in vivo", Life Sci Alliance, 6(12):e202302164, PMID: 37788908, DOI: 10.26508/isa.202302164 (link)
7. Ullah, T.R. et al., (2023), "Pharmacological inhibition of TBK1/IKKε blunts immunopathology in a murine model of SARS-CoV-2 infection", Nat Commun. 14(1):5666, PMID: 37723181, DOI: 10.1038/s41467-023-41381-9 (link)
8. Arbore, G. et al., (2023), "Pre-existing immunity drives the response to neoadjuvant chemotherapy in esophageal adenocarcinoma", Cancer Res., CAN-23-0356, PMID: 37350667, DOI: 10.1158/0008-5472.CAN-23-0356 (link)
9. Rajamanickam, A. et al., (2022), "Restoration of dendritic cell homeostasis and Type I/Type III interferon levels in convalescent COVID-19 individuals, BMC Immunol., 23(1):51, PMID: 36289478, DOI: 10.1186/s12865-022-00526-z (link)
10. Nagaoka, N., et al., (2022), "Effect of Casirivimab/Imdevimab Treatment on Serum Type I Interferon Levels in SARS-CoV-2 Infection", Viruses, 14(7):1399, DOI: 10.3390/v14071399 (link)
11. Dickey, L. et al., (2022), HIV-1-induced type I IFNs promote viral latency in macrophages, J. Leukoc. Biol. PMID:35588262, DOI:10.1002/JLB.4MA0422-616R (link)
12. Steiner, A. et al., (2022), Deficiency in coatomer complex I causes aberrant activation of STING signalling, Nature Communications, 13:2321, PMID: 35484149, DOI: 10.1038/s41467-022-29946-6 (link)
13. Akoi, Y. et al., (2022), Evaluation of the Relationships between Intestinal Regional Lymph Nodes and Immune Responses in Viral Infections in Children, Int. J. Mol. Sci., 23:318, DOI: 10.3390/ijms23010318 (link)
14. Jablonska, A., et al., (2021), The TLR9 2848C/T Polymorphism Is Associated with the CMV DNAemia among HIV/CMV Co-Infected Patients, Cells, 10:2360, DOI: 10.3390/cells10092360, (link)
15. Dorgham, et al. (2021). Considering Personalized Interferon Beta Therapy for COVID-19. Antimicrobial Agents and Chemotherapy, 3 pgs. PMID: 21321205. (link)
16. Contoli, M. et al., (2021), Blood Interferon-a Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients, Front. Immunol., PMID: 33767713, DOI: 10.3389/fimmu.2021.648004 (link)
17. Terajima, H et al., (2021), N6-methyladenosine promotes induction of ADAR1-mediated A-to-I RNA editing to supress aberrant antiviral innate immune response, PLoS Biol., 19(7):e3001292, PMID:34324489 (link)
18. Blanco-Melo, D. et al., (2020), Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, 181(5):1036, PMID: 32416070, DOI: 10.1016/j.cell.2020.04.026 (link)
19. Dubey, et al. (2020). Specific protein-protein interactions limit the cutaneous iontophoretic transport of interferon beta-1B and a poly-ARG interferon beta-1B analogue. International Journal of Pharmaceutics, 6 pgs. PMID: no PMID. (link)
看到这儿,您心动了吗?马上联系小艾吧!
美国的PBL Assay Science(又名:Pestka Biomedical Laboratories, Inc.)成立于1990年,创始人Sidney Pestka被称为“干扰素之父”,PBL Assay Science 作为干扰素和细胞因子蛋白和抗体以及预包装的一流的干扰素和细胞因子免疫测定试剂盒的高质量制造商而享有盛誉。生产和销售的高品质干扰素产品和生物标志物检测试剂盒在很多高影响力出版物中都有引用,并已在具有挑战性的样本中进行了外部验证。
PBL Assay Science提供各种干扰素亚型的蛋白,抗体和检测试剂盒,如:IFN-Alpha(IFN-ɑ 2a,IFN-ɑ 2b,IFN-ɑ 5,IFN-ɑ 6,IFN-ɑ 7,IFN-ɑ 14,IFN-ɑ 16,IFN-ɑ 17......),IFN-Beta(IFN-β 1a,IFN-β 1b),IFN-Lambda(IFN-λ),IFN-Omega(IFN-ω),IFN-Gamma(IFN-γ)......
武汉艾美捷科技有限公司,简称艾美捷,拥有进出口资质和自己的国际物流,与国内外90余家知名的试剂、原料及技术服务供应商深度合作,是国内生物制药企业、诊断企业、跨国药企、各大高校院所采购平台的首选定供应商。

微信扫码在线客服